Super fact 49 : The top one-meter (3.3 feet) of a typical 10 meters (33 feet) by 40 meters (131 feet) garden contains 2 kilograms (4.4 pounds) of Uranium. For comparison, the Hiroshima bomb contained 64 kilograms (121 pounds) of Uranium. Certain rocks such as Granite and Shale contain much more Uranium than soil. Uranium also exists in the atmosphere and there is 4.5 billion tons of Uranium in the ocean.
The numbers above come from the IAEA (International Atomic Energy Agency) and Stanford University . I should mention that the numbers vary depending on Geography, type of soil, etc. For example, there is much less Uranium in the soil in Florida compared to the soil in the Midwest.
This may come as a surprise to many people. Isn’t Uranium radioactive? How come we are still alive? That’s why I call this a super fact. The answer is that even though Uranium is used in nuclear bombs and nuclear reactors, it is by itself not very radioactive. You can hold natural uranium in your hand without much risk. The radioactivity from, for example, nuclear explosions come mainly from the fission process and the radioactivity from nuclear reactor waste is mainly from other isotopes created by the fission process in the reactor rather than the uranium itself.

What Are Isotopes?
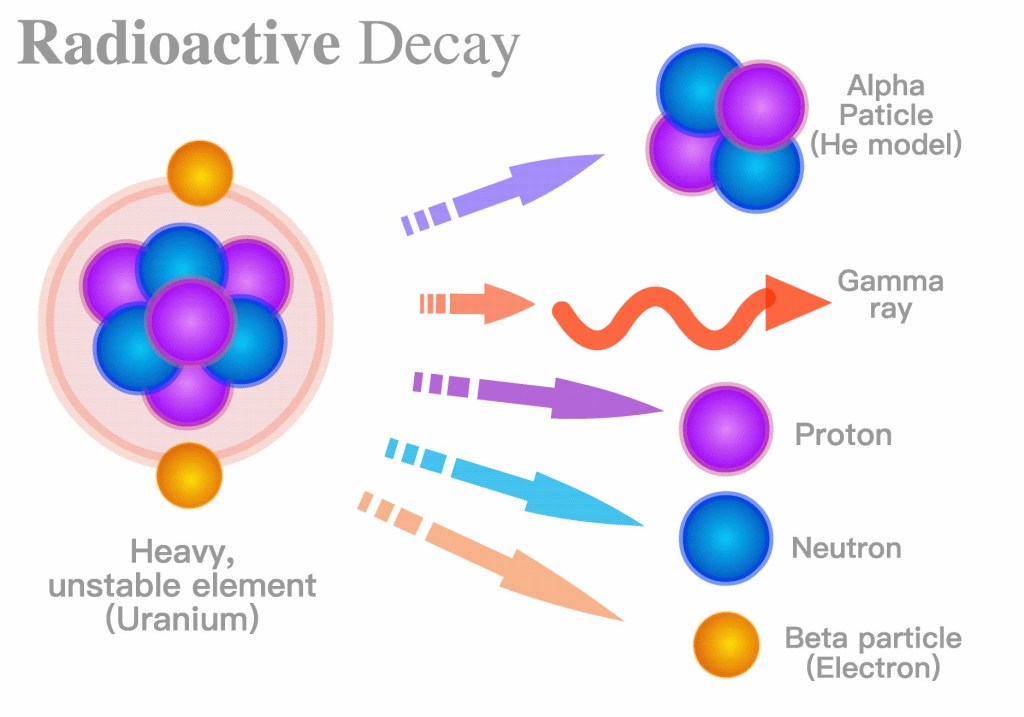
Before I explain some facts about the radioactivity and decay rate of Uranium, I should explain what an isotope is. Atoms consist of a nucleus and electrons surrounding the nucleus. In the nucleus there are protons and neutrons (and some other stuff). Neutral atoms have an equal amount of electrons and protons, which determines what kind of element it is. Hydrogen has one electron and one proton. Helium has two electrons and two protons. Oxygen has eight electrons and eight protons, etc. The number of protons/electrons is called the atomic number of the element.
The number of protons plus the number of neutrons is called the mass number. Atoms of the same element but different number of neutrons are called isotopes. Uranium-235 or U-235 has 92 protons and 235 – 92 = 143 neutrons. The number if protons/electrons determine the chemical properties of the element. The number of neutrons determines nuclear properties such as the stability of the nucleus, radioactivity, etc., as well as the weight. Therefore U-238 and U-235 are identical chemically and look and feel the same, but U-235 is more radioactive, and you can use U-235 for fission but not U-238.

The decay rate of Uranium
There are three main Uranium isotopes. Uranium-234, Uranium-235, and Uranium-238. Uranium-238 is the most common. 99.28% of natural Uranium is Uranium-238, 0.72% is Uranium-235 and 0.0057% is Uranium-234. Uranium-235 is the isotope we use for nuclear weapons.
The different isotopes have different decay rates and different levels of radioactivity. The half life of a radioactive isotope is the time it takes for an isotope to decay so that only half of it is left. The half-life of Uranium-238 is four and half billion years. That means that it will be around for a very long time, but since its decay rate is so slow, it is not very radioactive. The half-life of Uranium-235 is 710 million years, again it will be around for a very long time, but again, since its decay rate is so slow, it is not very radioactive. The half-life Uranium-234 is 247,000 years, a little bit faster but it still has a pretty slow decay rate.
This should be compared to Cesium-137, which has a half-life of roughly 30 years. In other words, it decays 150 million times faster than Uranium-238 and 23.7 million times faster than Uranium-235. Since Cesium-137 decays so much faster than the Uranium isotopes it means that each atom of Cesium-137 will send out radioactive particles much more often than a Uranium atom will, making it much more radioactive.
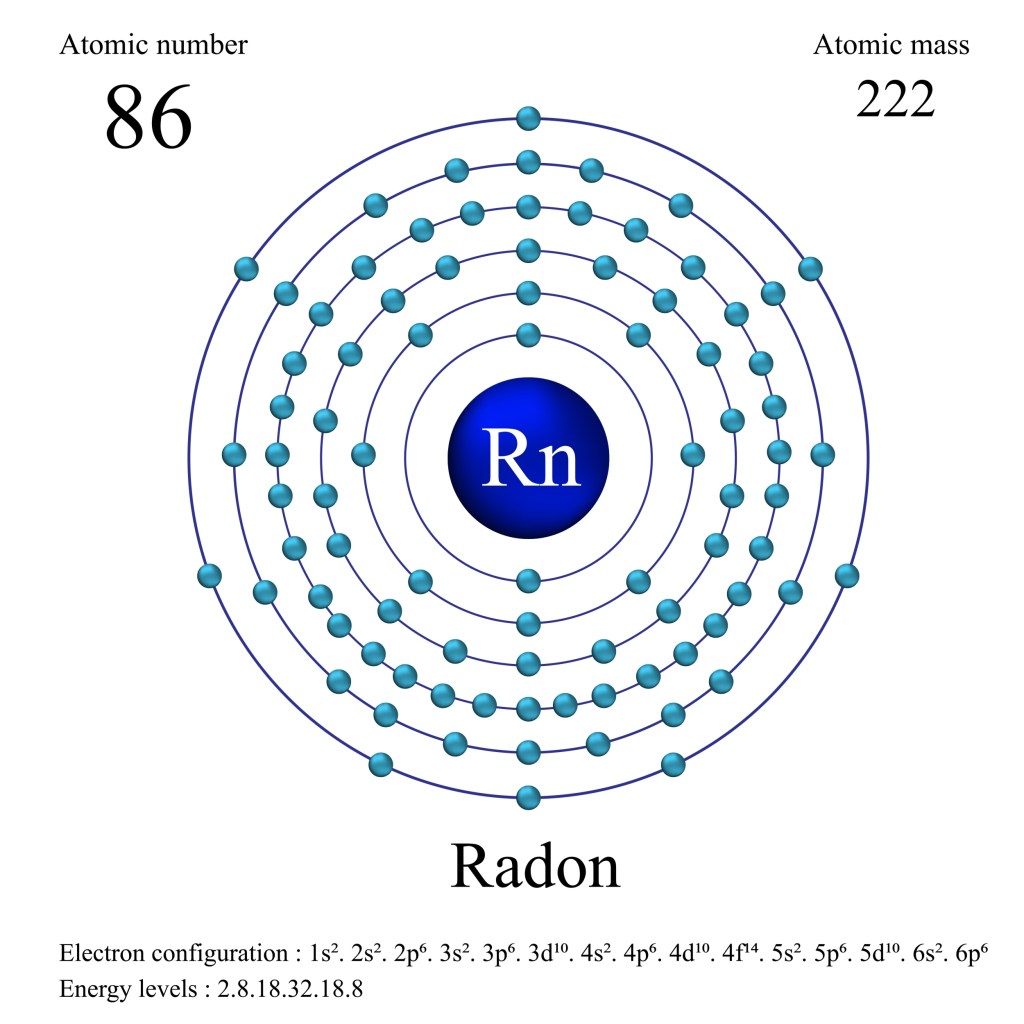
If you want to read about when I was walking around a whole day with a Cesium-137 sample in the back pocket of my jeans, click here. Radon-222, an extremely radioactive isotope of radon, which seeps into our basements from the inside of earth. It has a half-life of 3.82 days giving it a decay rate that is 430 billion times faster than Uranium-238 and 68 billion times faster than Uranium-235.
What makes it possible to make a nuclear bomb from Uranium-235 is not because it is very radioactive. It is not. It is because it has properties that make it perfect for bomb making. Each nucleus emits more than one neutron, in fact more than two on average, and the neutrons colliding with other Uranium-235 nucleuses can be made to travel at the correct speed to cause fission. In other words, it is fissile. It is a goldilocks situation. It is just right. Below is an illustration showing a chain reaction. Observe, the picture indicates that Uranium has 95 protons. This is wrong. Uranium has 92 protons. When I have the time, I will fix this picture.

Other Nuclear Related Posts
- Radon Represents our Largest Exposure to Ionizing Radiation.
- Ukraine Gave up Thousands of Nuclear Warheads.
- We Exploded Thousands of Nuclear Bombs.
- Review of Atomic Awakening.