Super fact 82 : All known cellular life descends from a single Last Universal Common Ancestor (LUCA). All animals, all plants, fungi, algae, green and red algae, kelp, phytoplankton, cyanobacteria, amoebas, amoebozoa, diatoms, stramenopiles, rhizaria, hacrobia, all eukaryote, all archaea, all bacteria, all the millions of species on Earth come from one single ancestor known as the Last Universal Common Ancestor – LUCA. Viruses are an exception, but viruses are not considered life.

LUCA, the Last Universal Common Ancestor, was not the first life form. It was preceded by earlier, simpler life forms that did not survive. LUCA was a single-celled, bacteria-like microorganism that existed roughly 4.2 billion years ago, or about 400 million years after planet Earth first formed. It was the final common ancestor for all currently living organisms. It thrived near hydrothermal vents as part of a larger microbial community before the three domains of life bacteria, archaea, and Eukarya diverged. This is a super fact because it is true, or at least highly probable, it is surprising and amazing and kind of important.
How Do We Know All Life Has a Common Ancestor ?
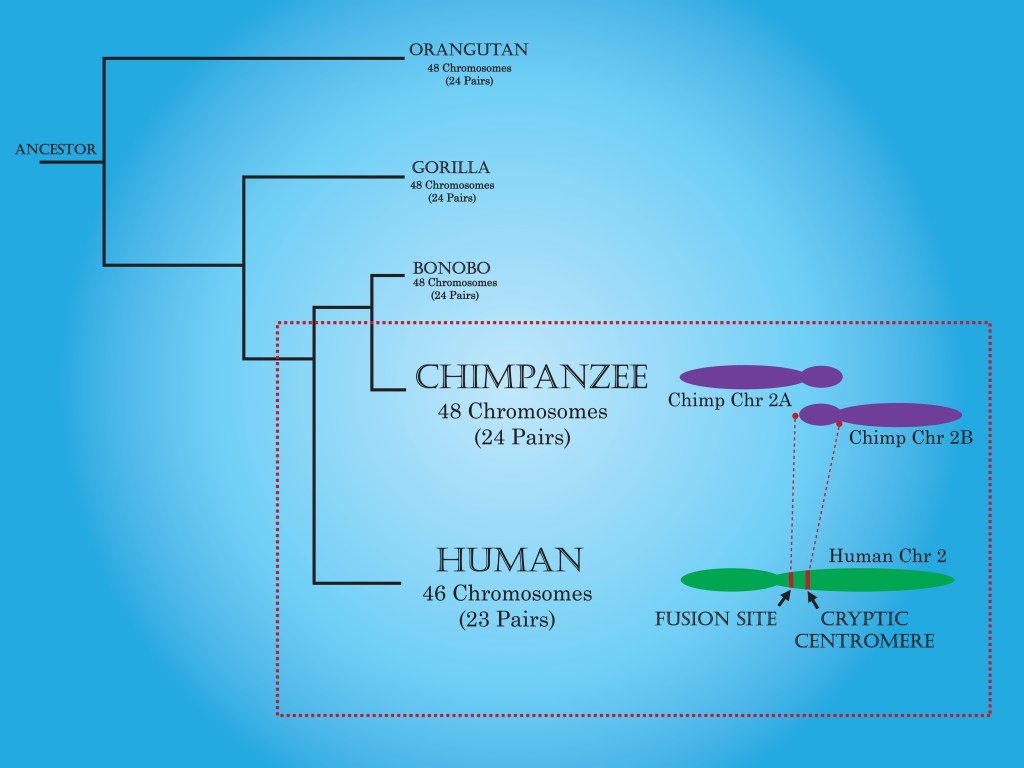
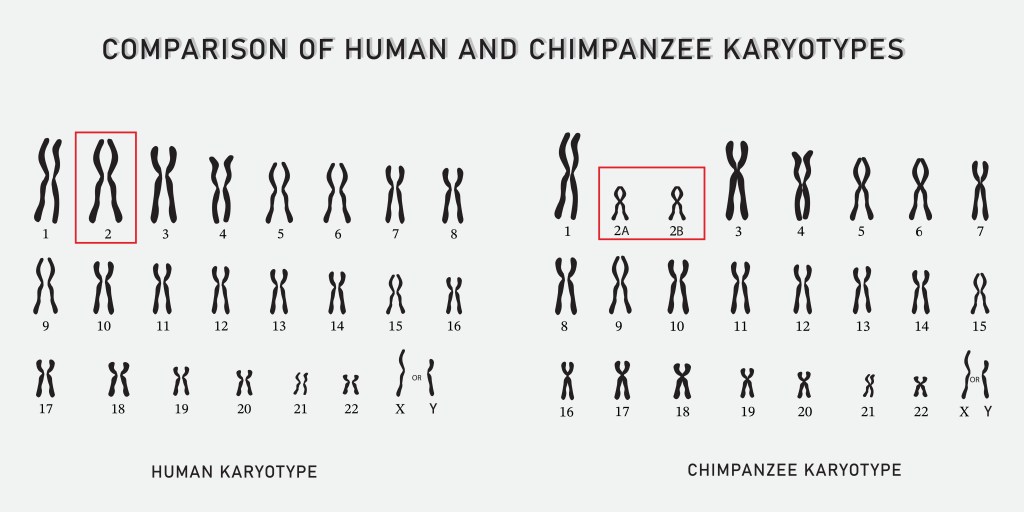
The answer is genome mining. By surveying nearly 2000 genomes of modern microbes we not only know that all life has a common ancestor (LUCA), that lived roughly 4.2 billion years ago, but we also know that it thrived near hydrothermal vents as part of a larger microbial community. This is analogous to another of my posts “Humans and Chimpanzees Have a Common Ancestor”. By sequencing human DNA and chimpanzee and bonobo DNA we know that humans and chimpanzees have a common ancestor. No fossils, or other information from the past is needed. DNA is a great tool for determining relationships between species and for finding information about past life, without the need of fossils.
To be more specific, the detailed biochemical similarity of all current life makes the existence of LUCA widely accepted by biochemists. There is a Universal Genetic Code, which means that nearly all living things use the same DNA/RNA-based genetic code to translate genetic information into proteins. There is a shared molecular machinery, for example, all life relies on ribosomes for protein synthesis, similar energy carriers like ATP, and the same 20 amino acids. All life uses the same mirror-image form of molecules, a signature of a single, common ancestry. In addition, there is a “core” set of 355 gene families present in both modern bacteria and archaea, which were likely inherited from LUCA. Finally, we have phylogenetic mapping, protein-sequence-based phylogenetic trees converge on a single root, indicating a common ancestry for all life. See the phylogenetic tree of life below.
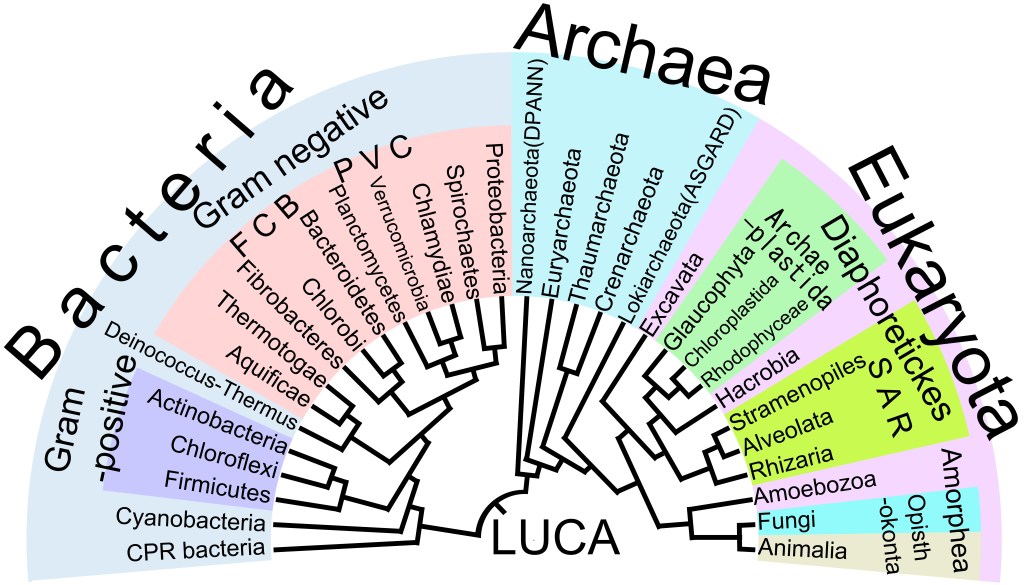
Below is another view of the diversification of life that focuses on the inventions made by life.
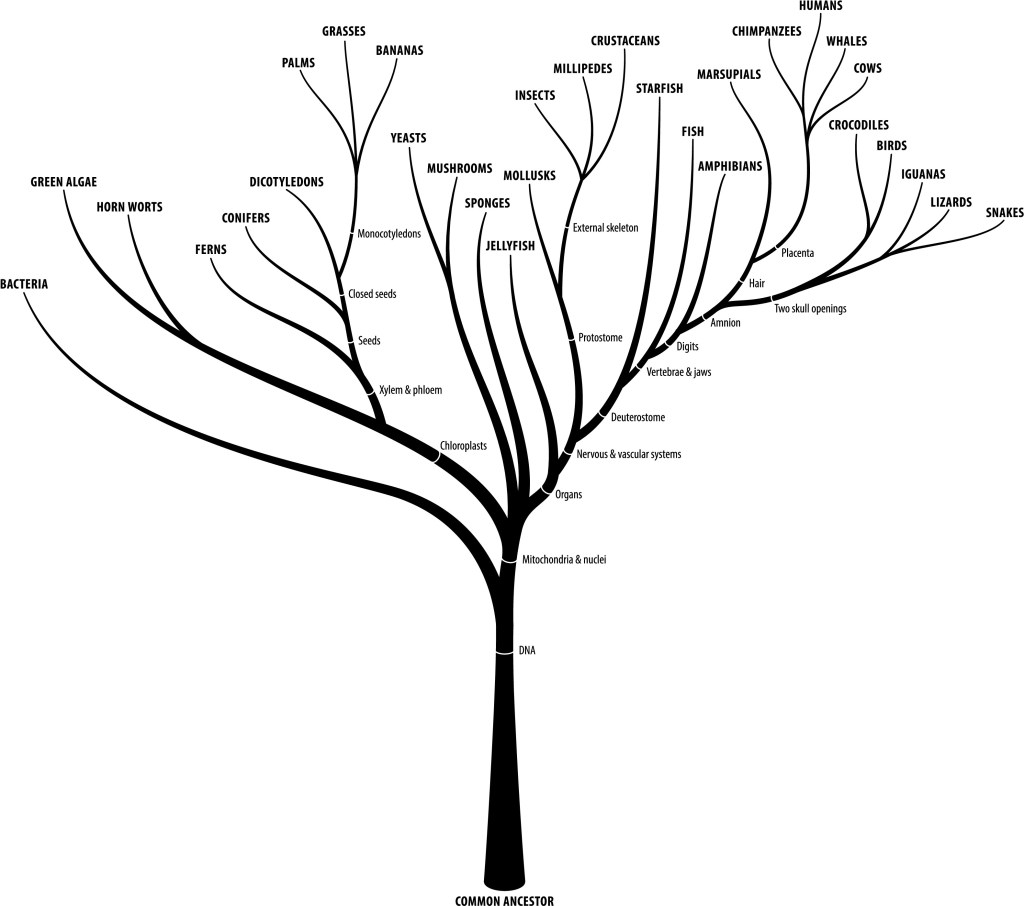
It should be noted that in addition to viruses there were likely other forms of life that existed alongside LUCA or before it. There was likely non-cellular life as well as cellular life that died out, RNA-based life, self-replicating nucleic acids, etc. It should also be noted that if some of the large viruses were to be reclassified as life, or a life form not based on LUCA were to be discovered then our “current LUCA” would no longer be LUCA, but just the ancestor of “almost all life”. That would still be amazing, just slightly less so.
The existence of LUCA brings up an interesting question. What would happen if we found DNA based life on another planet and its DNA showed that it also originated from LUCA ?
Other Evolution Related Super Facts
- Neanderthals Never Lived in Africa
- Evolution is a Fact
- The Second Law of Thermodynamics Does Not Contradict Evolution
- Humans and Chimpanzees Have a Common Ancestor